Home > Offer to Sell > Adhesives and Sealants > Adhesives and Sealants for Transportation > 1,4-Benzoquinone
1,4-Benzoquinone
Inquiry
| Post Date: | Dec 02,2015 |
| Expiry Date: | May 30,2016 |
| Detailed Description: |
Cas No. :106-51-4
Specs:99%min Payment Method: T/T,L/C,O/A. 1,4-Benzoquinone, commonly known as para-quinone, is a chemical compound with the formula C6H4O2. In a pure state, it forms bright-yellow crystals with a characteristic irritating odor, resembling that of chlorine, bleach, and hot plastic. This six-membered ring compound is the oxidized derivative of 1,4-hydroquinone. The molecule is multifunctional: it exhibits properties of a ketone, forming an oxime; an oxidant, forming the dihydroxy derivative; and an alkene, undergoing addition reactions, especially those typical for α,β-unsaturated ketones. 1,4-Benzoquinone is sensitive toward both strong mineral acids and alkali, which cause condensation and decomposition of the compound. Industrial routes 1,4-Benzoquinone is prepared by oxidation of diisopropylbenzene via a reaction related to the Hock rearrangement: C6H4(CHMe2)2 + 3 O2 → C6H4O2 + 2 OCMe2 + H2O The reaction proceeds via the bis(hydroperoxide). Acetone is a coproduct. Another major process involves the direct hydroxylation of phenol by acidic hydrogen peroxide: C6H5OH + H2O2 → C6H4(OH)2 + H2O Both hydroquinone and catechol are produced. Subsequent oxidation of the hydroquinone gives the quinone. Quinone was originally prepared industrially by oxidation of aniline, for example by manganese dioxide.This method is mainly practiced in PRC where environmental regulations are more relaxed. Applications Quinone is mainly used as a precursor to hydroquinone, which is used in photography and rubber manufacture as a reducing agent and antioxidant. Organic synthesis It is used as a hydrogen acceptor and oxidant in organic synthesis. 1,4-Benzoquinone serves as a dehydrogenation reagent. It is also used as a dienophile in Diels Alder reactions. Benzoquinone reacts with acetic anhydride and sulfuric acid to give the triacetate of hydroxyquinol. This reaction is called the Thiele-Winter reaction after Johannes Thiele, who first described it in 1898, and after Ernst Winter, who further described its reaction mechanism in 1900. An application is found in total d. Metabolism 1,4-Benzoquinone is a toxic metabolite found in human blood and can be used to track exposure to benzene or mixtures containing benzene and benzene compounds, such as petrol. The compound can interfere with cellular respiration, and kidney damage has been found in animals receiving severe exposure. It is excreted in its original form and also as variations of its own metabolite, hydroquinone. Benzoquinone compounds are a metabolite of paracetamol. 【Name】 1,4-Benzoquinone 【CAS】 106-51-4 【Synonyms】 1,4-BENZOCHINONE 1,4-BENZOQUINONE 2,5-CYCLOHEXADIENE-1,4-DIONE CYCLOHEXADIENEDIONE 1,4-Benzochinon 1,4-Benzoquine 1,4-Cyclohexadiene dioxide 1,4-Cyclohexadienedione 1,4-cyclohexadienedioxide 【EINECS】 203-405-2 【Molecular Formula】 C6H4O2 【Molecular Weight】 108.09 【Appearance】 gold powder 【mp 】 113-115 °C(lit.) 【bp 】 293°C 【density 】 1.31 【vapor density 】 3.73 (vs air) 【vapor pressure 】 0.1 mm Hg ( 25 °C) 【refractive index 】 n20/D 1.453 【Fp 】 38°C 【storage temp. 】 2-8°C 【Stability:】 Stable, but light sensitive. Incompatible with strong oxidizing agents. Flammable. 【Water Solubility 】 10 g/L (25 ºC) 【Hazard Codes 】 T,N,Xn,F 【Risk Statements 】 R23/25:Toxic by inhalation and if swallowed . R36/37/38:Irritating to eyes, respiratory system and skin . R50:Very Toxic to aquatic organisms. R20/21/22:Harmful by inhalation, in contact with skin and if swallowed . R11:Highly Flammable. 【Safety Statements 】 S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . S28:After contact with skin, wash immediately with plenty of ... (to be specified by the manufacturer) . S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . S61:Avoid release to the environment. Refer to special instructions safety data sheet . S23:Do not breathe gas/fumes/vapor/spray (appropriate wording to be specified by the manufacturer) . S16:Keep away from sources of ignition - No smoking . 【F 】 8 【HazardClass 】 6.1 【PackingGroup 】 II 【Usage 】 As dye intermediates, it can be used to analyse the determination of amino acids. Used in the qualitative assay of Coniine, pyridine, azoles, tyrosine and hydroquinone. It can also be used as quenchers of superoxide radical in the photocatalytic reaction, to speculate activity of the species in the process of photocatalytic, to infer the reaction mechanism. |
| CAS Registry Number: | 106-51-4 |
| Synonyms: | ;Quinone;p-Benzoquinone;Benzoquinone;p-Benzoquinone;P-quinone;PBQ;cyclohexa-2,5-diene-1,4-dione;cyclohexa-3,5-diene-1,2-dione; |
| Molecular Formula: | C6H4O2 |
| Molecular Weight: | 108.0948 |
| Molecular Structure: | 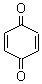
|
| Hazard Symbols: |  T:Toxic; T:Toxic; N:Dangerous for the environment; N:Dangerous for the environment; |
| Risk Codes: | R23/25:; R36/37/38:; R50:; |
| Safety Description: | S26:; S28A:; S45:; S61:; |
| Company: | TIANJIN ZHONGXIN CHEMTECH CO.,LTD. [ China ] |
| Contact: | sales(at)tjzxchem.com |
| Tel: | 86-22-89880739 |
| Fax: | 86-22-66880086 |
| Email: | sales@tjzxchem.com |
-
Disclaimer statement:The information and data included above have been realized by the enterprises and compiled by the staff, and are subject to change without notice to you. The Chemnet makes no warranties or representations whatsoever regarding the facticity, accuracy and validity of such information and data. In order to ensure your interest, we suggest you chose the products posted by our gold suppliers or VIP members.