Misoprostol
Inquiry
| Post Date: | Sep 10,2017 |
| Expiry Date: | Mar 09,2018 |
| Detailed Description: |
Cas No. :59122-46-2;62015-39-8
Payment Method: T/T, Western Union, MoneyGram Basic Info. Misoprostol CAS:59122-46-2 Molecular Formula: C22H38O5 Molecular Weight: 382.60 Chemical Properties: White Solid Assay:99% Solubility: Soluble to 100 mM in Ethanol Safety: Poison by ingestion, intramuscular, and intraperitoneal routes. When heated to decomposition it emits acrid smoke and irritating fumes. Package: Aluminium foil bag packing Storage: Keep in cool and dry place, away from light Misoprostol Description: Misoprostol is a medication used to start labor, induce abortions, prevent and treat stomach ulcers, and treat postpartum bleeding due to insufficient contraction of the uterus. For abortions it is used with mifepristone or methotrexate. It is a synthetic prostaglandin E1 (PGE1). Common side effects include diarrhea and abdominal pain. It is pregnancy category X meaning that it is known to result in negative fetal outcomes if taken during pregnancy. Uterine rupture may occur. It is on the World Health Organization's List of Essential Medicines, the most important medications needed in a basic health system. Misoprostol was invented and marketed by G.D. Searle & Company under the trade name Cytotec, but other trade names and generic formulations are available. Misoprostol Usage Misoprostol is approved for use in the prevention of NSAID-induced gastric ulcers. It acts upon gastric parietal cells, inhibiting the secretion of gastric acid by G-protein coupled receptor-mediated inhibition of adenylate cyclase, which leads to decreased intracellular cyclic AMP levels and decreased proton pump activity at the apical surface of the parietal cell. Because other classes of drugs, especially H2-receptor antagonists and proton pump inhibitors, are more effective for the treatment of acute peptic ulcers, misoprostol is only indicated for use by people who are both taking NSAIDs and are at high risk for NSAID-induced ulcers, including the elderly and people with ulcer complications. Misoprostol is sometimes coprescribed with NSAIDs to prevent their common adverse effect of gastric ulceration (e.g. with diclofenac in Arthrotec). Misoprostol has other protective actions, but is only clinically effective at doses high enough to reduce gastric acid secretion. For instance, at lower doses, misoprostol may stimulate increased secretion of the protective mucus that lines the gastrointestinal tract and increase mucosal blood flow, thereby increasing mucosal integrity. However, these effects are not pronounced enough to warrant prescription of misoprostol at doses lower than those needed to achieve gastric acid suppression. However, even in the treatment of NSAID-induced ulcers, omeprazole proved to be at least as effective as misoprostol,but was significantly better tolerated, so misoprostol should not be considered a first-line treatment. Misoprostol-induced diarrhea and the need for multiple daily doses (typically four) are the main issues impairing compliance with therapy. The most commonly reported adverse effect of taking a misoprostol orally for the prevention of stomach ulcers is diarrhea. In clinical trials, an average 13% of patients reported diarrhea, which was dose-related and usually developed early in the course of therapy (after 13 days) and was usually self-limiting (often resolving within 8 days), but sometimes (in 2% of patients) required discontinuation of misoprostol. The next most commonly reported adverse effects of taking misoprostol orally for the prevention of gastric ulcers are: abdominal pain, nausea, flatulence, headache, dyspepsia, vomiting, and constipation, but none of these adverse effects occurred significantly more often than when taking placebos. In practice, fever is almost universal when multiple doses are given every 4 to 6 hours. Misoprostol should not be taken by pregnant women to reduce the risk of NSAID-induced gastric ulcers because it increases uterine tone and contractions in pregnancy, which may cause partial or complete abortions, and because its use in pregnancy has been associated with birth defects. |
| CAS Registry Number: | 59122-46-2;62015-39-8 |
| Synonyms: | ;(11alpha,13E)-(+)-11alpha,16-Dihydroxy-16-methyl-9-oxoprost-13E-en-1-oic acid methyl ester;(+-)-Methyl (1R,2R,3R)-3-hydroxy-2-((E)-(4RS)-4-hydroxy-4-methyl-1-octenyl)-5-oxocyclopentaneheptanoate;(±)-15-Deoxy-(16RS)-16-hydroxy-16-methyl-PGE1 Methyl Ester;(±)-Methyl-(1R,2R,3R)-3-hydroxy-2-[(E)-(4RS)-4-hydroxy-4-methyl-1-octenyl]-5-oxocyclopentaneheptanoate;(11a,13E)-(±)-11,16-Dihydroxy-16-methyl-9-oxoprost-13-en-1-oic Acid Methyl Ester;methyl (11alpha,13E,16S)-11,16-dihydroxy-16-methyl-9-oxoprost-13-en-1-oate;methyl (11alpha,13E)-11-hydroxy-16,16-dimethyl-9-oxoprost-13-en-1-oate; |
| Molecular Formula: | C23H40O4 |
| Molecular Weight: | 380.5613 |
| Molecular Structure: | 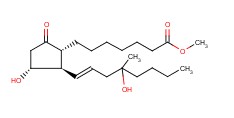
|
| Hazard Symbols: |  T:Toxic; T:Toxic; |
| Risk Codes: | R60:; R61:; R25:; |
| Safety Description: | S53:; S22:; S36/37/39:; S45:; |
| Company: | Guangzhou Kafen Biotech Co.,Ltd [ China ] |
| Contact: | laura Lin |
| Tel: | +8613332873040 |
| Fax: | |
| Email: | laura@rawroid.com |
-
Disclaimer statement:The information and data included above have been realized by the enterprises and compiled by the staff, and are subject to change without notice to you. The Chemnet makes no warranties or representations whatsoever regarding the facticity, accuracy and validity of such information and data. In order to ensure your interest, we suggest you chose the products posted by our gold suppliers or VIP members.