| Detailed Description: |
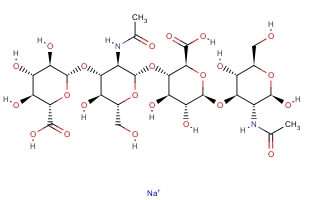
Cas No. :9067-32-7
Pharmaceutical Grade of HA
Test Items |
Eyes-drop Grade |
Injection Grade |
Description |
White, fine powder |
White, fine powder |
pH |
6.0-7.5 |
6.0-7.5 |
Assay |
≥91.0% |
≥91.0% |
Transparency |
≥99.0% |
≥99.0% |
Molecular Weight |
≥1.00×10 6 |
≥2.00×10 6 |
Glucuronic Acid |
≥44.0% |
≥44.0% |
Protein |
≤0.1% |
≤0.1% |
Heavy Metals |
≤20ppm |
≤20ppm |
Arsenic |
≤2ppm |
≤2ppm |
Loss on Drying |
≤10.0% |
≤10.0% |
Nitrogen |
3.0-4.0% |
3.0-4.0% |
Intrinsic Viscosity |
≥18.0dL/g |
≥30.0dL/g |
Bacterial Endotoxin |
≤0.5IU/mg |
≤0.05IU/mg |
Bacteria Count |
<10cfu/g |
<10cfu/g |
Mold and Yeast |
<10cfu/g |
<10cfu/g |
Staphylococcus Aureus |
Negative |
Negative |
Pseudomonas Aeruginosa |
Negative |
Negative |
|