Sodium carbonate CAS:497-19-8
Inquiry
| Post Date: | Jun 27,2018 |
| Expiry Date: | Dec 24,2018 |
| Detailed Description: |
Cas No. :497-19-8;7542-12-3
Quantity: 50000Metric Tons Specs:99% Price:300 USD Metric Tons Payment Method: L/C,T/T Sodium carbonate Product name:Sodium carbonate Other names:Soda ash, Washing soda, Soda crystals CAS:497-19-8 (anhydrous) Yes 5968-11-6 (monohydrate) 6132-02-1 (decahydrate) EC Number:207-838-8 MF:Na2CO3 Description: Sodium carbonate, Na2CO3, (also known as washing soda, soda ash and soda crystals, and in the monohydrate form as crystal carbonate) is the water-soluble sodium salt of carbonic acid. It most commonly occurs as a crystalline decahydrate, which readily effloresces to form a white powder, the monohydrate. Pure sodium carbonate is a white, odorless powder that is hygroscopic (absorbs moisture from the air). It has a strongly alkaline taste, and forms a moderatesly basic solution in water. Application: It is used as a pH regulator to maintain stable alkaline conditions necessary for the action of the majority of photographic film developing agents. It acts as an alkali because when dissolved in water, it dissociates into the weak acid: carbonic acid and the strong alkali: sodium hydroxide. This gives sodium carbonate in solution the ability to attack metals such as aluminium with the release of hydrogen gas.[13] It is a common additive in swimming pools used to raise the pH which can be lowered by chlorine tablets and other additives which contains acids. In cooking, it is sometimes used in place of sodium hydroxide for lyeing, especially with German pretzels and lye rolls. These dishes are treated with a solution of an alkaline substance to change the pH of the surface of the food and improve browning. In taxidermy, sodium carbonate added to boiling water will remove flesh from the bones of animal carcasses for trophy mounting or educational display. In chemistry, it is often used as an electrolyte. Electrolytes are usually salt-based, and sodium carbonate acts as a very good conductor in the process of electrolysis. In addition, unlike chloride ions, which form chlorine gas, carbonate ions are not corrosive to the anodes. It is also used as a primary standard for acid-base titrations because it is solid and air-stable, making it easy to weigh accurately. |
| CAS Registry Number: | 497-19-8;7542-12-3 |
| Synonyms: | ;Sodium carbonate anhydrous;Sodium carbonate solution;soda ash;disodium carbonate;Sodium carbonate-12C, 13C-depleted;calcined soda;Carbonic acid disodium salt;Sodium carbonate,high-purity;Sodium carbonate,dense;SODA ASH LIGHT;Anhydrous Sodium carbonate;carbonic acid, sodium salt;Carbonic acid, sodium salt;Caswell No. 752;Soda (VAN);soda ash dense;Soda;Sodium carbonate,anhydrous; |
| Molecular Formula: | Na2CO3 |
| Molecular Weight: | 105.99 |
| Molecular Structure: | 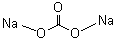
|
| Hazard Symbols: |  Xi:Irritant; Xi:Irritant; |
| Risk Codes: | R36:; |
| Safety Description: | S22:; S26:; |
| Company: | Taian Guangyuan International Trade Co LTD [ China ] |
| Contact: | Angela |
| Tel: | +8618754801539 |
| Fax: | |
| Email: | angela@chemcola.com |
-
Disclaimer statement:The information and data included above have been realized by the enterprises and compiled by the staff, and are subject to change without notice to you. The Chemnet makes no warranties or representations whatsoever regarding the facticity, accuracy and validity of such information and data. In order to ensure your interest, we suggest you chose the products posted by our gold suppliers or VIP members.