98% AMMONIUM BIFLUORIDE
-
Post Date:
Dec 19,2013 -
Expiry Date:
Jun 17,2014 -
Detailed Description:
Cas No. :1341-49-7 Quantity: 5000Metric Tons
Specs:40%
Price:800 - 900 USD Metric Tons
Payment Method: L/C,T/T,Western Union
Specifications
Ammonium bifluoride is a corrosive chemical, it is easy deliquescence and can dissolve glass
National Standard Code: 83003
CAS: 1341-49-7
EINECS: 215-676-4
UN CODE: 1727
Hazard Lable: None
Physical and Chemical Properties:
Ammonium bifluoride is a corrosive chemical, it is easy deliquescence and can dissolve glass.
Chemical Formula:(NH4)HF2
Molecular Weight: 57.04
Melting Point: 125.6°C
Boiling Point: No data
Solubility: easily soluble in water, slightly soluble in alcohols
Saturated Vapor Pressure(kPa):No data
Relative Density(water=1):1.5
Stability: Deliquescent
National Standard GB/T 1278-94:
Name
Analytical Reagent
Chemical reagent
Residue of burning
(in sulfate)0.01
0.05
Chloride(Cl)
0.001
0.005
Sulfate (SO4)
0.005
0.01
Fluoroborate(in SiF6)
0.2
0.5
Feterrum(Fe)
0.001
0.005
Heavy metal (in Pb)
0.002
0.005
Main usage:
Ammonium bifluoride is corrosive to glass and skin. It can be used as chemical reagent, glass etchant (together with hydrofluoric acid), disinfectant and preservatives in fermentation industry, solvent for metal beryllium preparation with beryllium oxide and surface treating agent for silicon steel plate, it can also be used to manufacture ceramic, magnesium alloy, cleaning and descaling of boiler feed system and vapor generating system as well as acidation of oil sand, as one component of alkylation, isomerization catalyst. It is used to refine beryllium, make electric welding rod, cast steel, preserve wood etc.
Emergency Measures:
Inhalation: Remove victim to fresh air. Keep the respiratory tract unobstructed. If breathing is difficult, give oxygen. If not breathing, give artificial respiration.
Ingestion: Gargle with water, give milk or water.
Skin Contact: Discard contaminated clothing and flush with flowing water for at least 15 min, and get medical aid; or Discard contaminated clothing and flush with diphoterine or hexaflourine(for fluorin-containing acid) .
-
CAS Registry Number:
1341-49-7 -
Synonyms:
;Ammonium hydrogen difluoride;ammoniumfluoridecomp.withhydrogenfluoride(1:1);ammoniumfluoridecomp withhydrogenfluoride(1:1);ammoniumbifluoride,solution;ammoniumfluoride((nh4)(hf2));AMMONIUM HYDROGEN FLUORIDE;acidammoniumfluoride;ammoniumbifluoride,solid;hydrofluoric acid, ammonium salt (1:1);Ammonium Hydrogenfluoride; -
Molecular Formula:
NH4HF2 -
Molecular Weight:
38.0443 -
Molecular Structure:
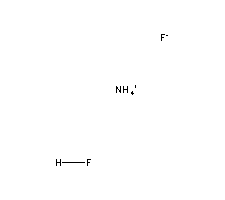
-
Hazard Symbols:
T:Toxic;
C:Corrosive;
-
Risk Codes:
R25:;
R34:;
-
Safety Description:
S22:;
S26:;
S37:;
S45:;
-
Company:
Shenyang Huijinfengda Chemical Co., Ltd. [ China ] -
Contact:
Amaris -
Tel:
+86-024-25121168 -
Fax:
+86-024-25126998 -
Email:
chemochina@gmail.com