HyaPolyTM Sodium Hyaluronate injection Grade
-
Post Date:
Jul 10,2013
-
Expiry Date:
Jan 06,2014
-
Detailed Description:
Cas No. :9067-32-7
Quantity: 100Kilograms
Specs:Injection Grade
Payment Method: T/T in advance
Product name: HyaPolyTM Sodium Hyaluronate injection Grade
Appearance: White or almost white powder or fibrous aggregate
Identification:
A Infrared absorption Consistent with the Ph.Eur.reference spectrum of sodium hyaluronate
B Reaction Positive
Assay (dried substance): 95.0%~105.0%
PH: 5.0 ~ 8.5
Intrinsic viscosity: 1.6~2.2 m3/kg
Nucleic acids: A260nm≤0.5
Protein ≤0.1%:
Loss on drying: ≤15.0%
Chlorides: ≤0.5%
Iron: ≤30ppm
Heavy metal: ≤10ppm
Bacterial endotoxins: <0.05 IU/mg
Total aerobic microbial count(TAMC): ≤100cfu/g
Residual solvets(Ethanol): ≤5000ppm
Storage Store in cool(2℃-10℃) away from moisture and direct sunlight.
Shelf life 2 years if sealed and stored properly.
-
CAS Registry Number:
9067-32-7
-
Synonyms:
;Hyaluronic acid sodium salt;HA Sodium;Hyaluronate Sodium;Oligomerie Sodium Hysluronate;
-
Molecular Formula:
C28H44N2O23·Na
-
Molecular Weight:
799.6366
-
Molecular Structure:
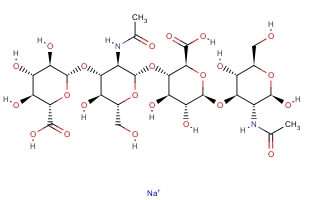
-
Safety Description:
S22:;
S24/25:;
-
Company:
Kangcare Bioindustry Co.,Ltd
[ China ]
-
Contact:
Joanna SHI
-
Tel:
+86-25-83283994
-
Fax:
+86-25-84650149
-
Email:
joanna.shi@kangcare.com
Inquiry
