Ledipasvir (GS-5885) CAS 1256388-51-8 (jerryzhang001@chembj.com)
-
Post Date:
Apr 20,2017
-
Expiry Date:
Apr 20,2018
-
Detailed Description:
Cas No. :1256388-51-8
Payment Method: T/T; Western Union; Moneygram and Bitcoin
Ledipasvir (GS-5885) CAS 1256388-51-8
Product Name: Ledipasvir (GS-5885)
Synonyms: GS 5885;GS5885;GS-5885;Ledipasvir;GS-5885/Ledipasvir;gs-5885/gs5885;Ledipasvir / GS 5885;GS 588
CAS: 1256388-51-8
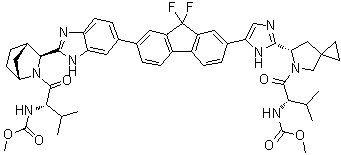
MF: C49H54F2N8O6
Molecular Weight: 889.0
Product Categories: API
Categories: NS5A inhibitors;Fluorenes;Carbamates;Benzimidazoles;Cyclopropanes;Imidazoles;Breakthrough therapy
Purity : 98%
Solubility DMSO
Storage at -20°C
Appearance : White powders
Package : 50g/foil bag
Usage : Ledipasvir is most commonly used in combination with sofosbuvir for treatment in chronic hepatitis C genotype 1 patients. This drug has been tested and shown efficacy in treatment-naive and treatment experienced patients.
Product Description :
Ledipasvir (formerly GS-5885) is a drug for the treatment of hepatitis C that was developed by Gilead Sciences.After completing Phase III clinical trials, on February 10, 2014 Gilead filed for U.S. approval of a ledipasvir/sofosbuvir fixed-dose combination tablet for genotype 1 hepatitis C. The ledipasvir/sofosbuvir combination is a direct-acting antiviral agent that interferes with HCV replication and can be used to treat patients with genotypes 1a or 1b without PEG-interferon or ribavirin.
Ledipasvir is an inhibitor of the hepatitis C virus NS5A protein.
Data presented at the 20th Conference on Retroviruses and Opportunistic Infections in March 2013 showed that a triple regimen of the nucleotide analog inhibitor sofosbuvir, ledipasvir, and ribavirin produced a 12-week post-treatment sustained virological response (SVR12) rate of 100% for both treatment-naive patients and prior non-responders with HCV genotype 1.The sofosbuvir/ledipasvir coformulation is being tested with and without ribavirin. In February 2014 Gilead has filed for United States Food and Drug Administration (FDA) approval of ledipasvir/sofosbuvir oral treatment, without interferon and ribavirin.
On October 10, 2014 the FDA approved the combination product ledipasvir 90 mg/sofosbuvir 400 mg called Harvoni.
Application :
GS-5885 is an inhibitor of the hepatitis C virus (HCV) NS5A protein and exhibits potent suppression of genotype 1 HCV replicons. GS-5885 was well tolerated and resulted in median maximal reductions in HCV RNA ranging from 2.3 log(10) IU/ml (1 mg QD) to 3.3 log(10) IU/ml (10 mg QD in genotype 1b and 30 mg QD). E(max) modeling indicated GS-5885 30 mg was associated with>95% of maximal antiviral response to HCV genotype 1a. HCV RNA reductions were generally more sustained among patients with genotype 1b vs. 1a. Three of 60 patients had a reduced response and harbored NS5A-resistant virus at baseline. NS5A sequencing identified residues 30 and 31 in genotype 1a, and 93 in genotype 1b as the predominant sites of mutation following GS-5885 dosing. Ledipasvir (formerly GS-5885) is currently in Phase III clinical trials.
-
CAS Registry Number:
1256388-51-8
-
Synonyms:
;Methyl[(2S)-1-{(6S)-6-[5-(9,9-Difluoro-7-{2-[(1R,3S,4S)-2-{(2S)-2[(methoxycarbonyl)amino]-3-methylbutanoyl}-2-azabicyclo[2.2.1]-hept-3-yl]-1H-benzimidazol-6-yl}-9H-fluoren-2-yl)-1H-imidazol-2-yl]-5-azaspiro[2.4]hept-5-yl}-3-methyl-1-oxobutan-2-yl]carba-mate;
-
Molecular Formula:
C49H54F2N8O6
-
Molecular Weight:
889.00
-
Molecular Structure:
-
Company:
Nanjing Bangnuo Biotechnology Co., Ltd.
[ China ]
-
Contact:
Jerry Zhang
-
Tel:
86-25-52198306
-
Fax:
-
Email:
jerryzhang001@chembj.com
Inquiry
