Levamisole hydrochloride cetirizine 130018-87-0
-
Post Date:
Jan 15,2019
-
Expiry Date:
Jan 15,2020
-
Detailed Description:
Cas No. :130018-87-0
Specification:
Items
Acceptance criteria
Results
Character
Appearance: White or almost white powder.
Almost white powder
Solubility: Slightly soluble in water, freely
soluble in dilute acetic acid and in methanol,
slightly soluble in alcohol.
Complies
Identification
A.Specific optical rotation: –30.0º~-33.5º
(dried substance)
–33.3º
B.Infrared absorption spectrophotometry:
The IR spectrum is consistent with that
obtained with Levodropropizine CRS.
Complies
Tests
pH
9.2~10.2
9.4
Impurity B and related
substances
Impurity B: ≤ 0.5%
≤ 0.1%
Any other impurity: ≤ 0.1 %
≤ 0.1%
Total impurities: ≤ 0.6 %
≤ 0.1%
Impurity C
≤ 5 ppm
≤ 5ppm
Enantiomeric purity
Impurity A: ≤ 2 %
0.2 %
Loss on drying
≤ 1.0 %.
≤ 0.1%
Sulphated ash
≤ 0.2 %
≤ 0.1%
Assay
98.5% ~ 101.0%(dried substance)
99.7%
Conclusion
The product meets the specification of EP7.0
-
CAS Registry Number:
130018-87-0
-
Synonyms:
;Leocetirizine Hydrochloride;Levocetirizine 2Hcl;Levocetirizine Dihcl;Levocetirizine Hcl;Levocetrizine Dihydrochloride;[2-[4-[(R)-(4-Chlorophenyl)Phenylmethyl]-1-Piperazinyl]Ethoxy]-Acetic Acid Dihydrochloride;Levocetrizinehcl;Levocitirizine Dihydrochloride;Levo Cetirzine Dihydrochloride;Levocitirizine Dihcl;4-{4-[(S)-(4-Chlorophenyl)(Phenyl)Methyl]Piperazin-1-Yl}Butanoic Acid Hydrochloride (1:1);Levocetirizine Hydrochloride;Levocetrizine Hydrochloride;Levocitirizine 2Hcl;
-
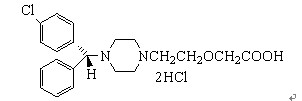
Molecular Formula:
C21H26Cl2N2O2
-
Molecular Weight:
409.34934
-
Molecular Structure:
-
Company:
Wuhan Benjamin Pharmaceutical Chemical Co.,Ltd
[ China ]
-
Contact:
Miss Wang
-
Tel:
027-50661278
-
Fax:
027-87569548
-
Email:
sales03@benjaminpharmchem.com
Inquiry
