Sodium Hyaluronate
-
Post Date:
Feb 07,2020
-
Expiry Date:
Feb 06,2021
-
Detailed Description:
Cas No. :9067-32-7
Descriptions
Hyaluronic acid is a polymeric mucopolysaccharide composed of D-N-acetylglucosamine and D-glucuronic acid as structural units.
Generally, commercial hyaluronic acid is present in the form of its sodium salt, namely sodium hyaluronate. It can increase the stability of the product and reduce the possibility of oxidation.
HyaluroNa™, produced by fermentation of Streptococcus zooepidemicus.
Specifications
HyaluroNa™ Cosmetic/Food Grade
HyaluroNa™ ULMW 3K Da~10K Da
HyaluroNa™ LMW 10K Da~0.2M Da
HyaluroNa™ MMW 0.2M Da~1.6M Da
HyaluroNa™ HMW 1.6M Da~3.0M Da
HyaluroNa™ Cation
HyaluroNa™ Oligosaccharide (<2K Da)
HyaluroNa™ 1% Solution
HyaluroNa™ Oil Dispersed
HyaluroNa™ Acetylated
HyaluroNa™ Eye Drop
Functions
Joint care
Natural moisturizing
Prevent the occurrence of arteriosclerosis, pulse disturbance and brain atrophy
Wound healing
Promote the proliferation and differentiation of epidermal cells, scavenge oxygen free radicals, prevent and repair skin damage
Applications
Dietary supplements
Cosmetics
Eye drops
Feed
Recommended Dosages
Creams, Emulsions, Lotions, Essences, Facial Cleansers, Body Lotions, Shampoo and Hair Extension Agents, Mousses, Lipsticks and Other Cosmetics, the general addition amount is 0.05-0.5%.
-
CAS Registry Number:
9067-32-7
-
Synonyms:
;Hyaluronic acid sodium salt;HA Sodium;Hyaluronate Sodium;Oligomerie Sodium Hysluronate;
-
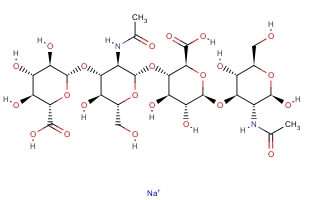
Molecular Formula:
C28H44N2O23·Na
-
Molecular Weight:
799.6366
-
Molecular Structure:
-
Safety Description:
S22:;
S24/25:;
-
Company:
AKMECHEM BIOTECH CO., LTD
-
Contact:
Mr. Shi
-
Tel:
0553-5624933
-
Fax:
-
-
Email:
info@akmechem.com
Inquiry
