Vonoprazan Fumarate
-
Post Date:
Sep 25,2025
-
Expiry Date:
Mar 24,2026
-
Detailed Description:
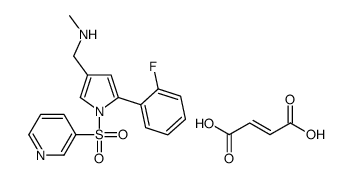
Cas No. :1260141-27-2
Vonoprazan fumarate (Takecab), discovered and developed by Takeda and Otsuka, was approved by the PMDA of Japan in December 2014, and is indicated for the treatment of gastric ulcer, duodenal ulcer and reflux esophagitis.
Vonoprazan fumarate is an oral, newly synthesised potassium-competitive acid blocker (P-CAB) with antisecretory activity. It is also a proton pump inhibitor (PPI) reversibly inhibiting H+/K+, ATPase. It is mainly used in the treatment of acid-related diseases such as GERD and peptic ulcer disease.
-
CAS Registry Number:
1260141-27-2
-
Synonyms:
;Vonoprazan fumarate;TAK-438;TAK 438;5-(2-Fluorophenyl)-N-methyl-1-(3-pyridinylsulfonyl)-1H-pyrrole-3-methanamine (2E)-2-butenedioate;Vonaprazan;TAK438;
-
Molecular Formula:
C21H20FN3O6S
-
Molecular Weight:
461.46300
-
Molecular Structure:
Inquiry
