Pitavastatin Calcium
Inquiry
| Post Date: | Apr 25,2024 |
| Expiry Date: | Oct 22,2024 |
| Detailed Description: |
Cas No. :147526-32-7
含量:≥98.5% CAS:147511-69-1 用途:降血酯药 ITEM STANDARD CHARACTER A WHITE OR ALMOST WHITE POWDER IDENTIFICATION POSITIVE REACTION SPECIFIC OPTICAL ROTATION () +14 - +22 PH 5.6 - 7.2 RELATED IMPURITIES (%) TOTAL IMPURITY(%) ≤1.0 INDIVIDUAL IMPURITY (%) ≤0.2 LOSS ON DRYING (%) ≤5.0 Ca (%) 3.5 - 4.5 HEAVY METALS (UG/G) ≤20 ASSAY (ON UNHYDROUS BASIS) (%) ≥98.5 |
| CAS Registry Number: | 147526-32-7 |
| Synonyms: | ;(+)-Monocalciumbis{(3R,5S,6E)-7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolyl]-3,5-dihydroxy-6-heptenoate};calcium bis{(1R,3R,5S,6E)-7-[2-cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl]-1,3,5-trihydroxyhept-6-en-1-olate};(+)-Monocalciumbis{(3R,5S,6E)-7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolyl]-,5-dihydroxy-6-heptenoate}; |
| Molecular Formula: | C50H48CaF2N2O8 |
| Molecular Weight: | 882.9995264 |
| Molecular Structure: | 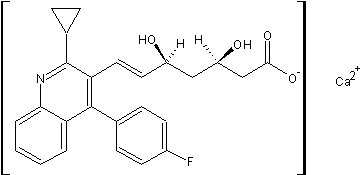
|
| Company: | Quzhou Aifeimu Chemical Co., Ltd. [ China ] |
| Contact: | David chen |
| Tel: | 86-570-6040288 |
| Fax: | 86-570-6040488 |
| Email: | sales@afmpharm.com |
-
Disclaimer statement:The information and data included above have been realized by the enterprises and compiled by the staff, and are subject to change without notice to you. The Chemnet makes no warranties or representations whatsoever regarding the facticity, accuracy and validity of such information and data. In order to ensure your interest, we suggest you chose the products posted by our gold suppliers or VIP members.

-
- C-2
- C-1
- (E)-3-[3-(4-Fluorophenyl)-...
- (4R-cis)-1,1-Dimethylethyl...
- Bis[(3R,5S,6E)-7-(2-Cyclop...
- (4R-Cis)-6-Hydroxymethyl-2...
- 4-(4-Fluorophenyl)-6-Isopr...
- (R)-4-Cyano-3-Hydroxybutyr...
- R-1-3
- Tert-butyl-(+)7-[4-(4-fluo...
- R-3
- N-1
- Ethyl-2-Cyclopropyl-4-(4-F...
- N-2
- ATS-8
- R-1-2 Methanol
- F-1
- (4R,Cis)-1,1-Dimethylethy-...
- (3R,5R)-7-[2-(4-Fluorophen...
- 3-(4-Fluorophenyl)-1-(1-Me...
- Tert-butyl(E)-3,5-Dihydrox...
- ATS-5
- Rosuvastatin Calcium
- (4R-Cis)-6-[(Acetyloxy met...
- Atorvastatin Sodium