- Category :
Fragrances and Aroma chemicals
- CAS NO : 80-59-1
- EC NO : 201-295-0
- Molecular Formula : C5H8O2
- Main Specifications : 98%min
- Synonyms : Tiglic acid;
Package: 25kg/drum
Uses : used as fragrances and flavors
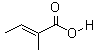
Molecular Structure:
Product description:
Tiglic acid Basic information
Product Name: Tiglic acid
Synonyms: (E)-2-METHYL-2-BUTENOIC ACID;E-2,3-DIMETHYLACRYLIC ACID;FEMA 3599;2,3-DIMETHYLACRYLIC ACID;2-METHYL-TRANS-2-BUTENOIC ACID;2-METHYL-2-BUTENOIC ACID;2-METHYLBUT-2-ENOIC ACID;2-METHYLCROTONIC ACID
CAS: 80-59-1
MF: C5H8O2
MW: 100.12
EINECS: 201-295-0
Product Categories: Aromatic Cinnamic Acids, Esters and Derivatives
Mol File: 80-59-1.mol
Tiglic acid Chemical Properties
mp 61-64 ℃(lit.)
bp 95-96℃ 12 mm Hg(lit.)
density 0.969 g/mL at 25 ℃(lit.)
FEMA 3599
Fp 101℃
storage temp. Refrigerator (+4℃)
Water Solubility SOLUBLE IN HOT WATER
Merck 14,9433
BRN 1236500
CAS DataBase Reference 80-59-1(CAS DataBase Reference)
NIST Chemistry Reference 2-Butenoic acid, 2-methyl-, (E)-(80-59-1)
EPA Substance Registry System 2-Butenoic acid, 2-methyl-, (2E)-(80-59-1)
Safety Information
Hazard Codes C,Xi
Risk Statements 34-36/37/38
Safety Statements 26-36/37/39-45-24/25-27
RIDADR UN 3261 8/PG 2
WGK Germany 2
RTECS GQ5430000
HazardClass 8
PackingGroup III
HS Code 29161980
Hazardous Substances Data 80-59-1(Hazardous Substances Data)
MSDS Information
Provider Language
Tiglic acid English
ACROS English
SigmaAldrich English
ALFA English
Tiglic acid Usage And Synthesis
Chemical Properties WHITE TO BEIGE CRYSTALLINE POWDER AND CHUNKS
General Description A white lustrous flaked solid with a fruity or spicy odor. About the same density as water and slightly soluble in water. May burn if heated to high temperatures. May severely irritate skin, eyes, lungs or mucous membranes.
Air & Water Reactions Slightly soluble in water.
Reactivity Profile TIGLIC ACID is a carboxylic acid. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. Even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in Tiglic acid to corrode or dissolve iron, steel, and aluminum parts and containers. Carboxylic acids, like other acids, react with cyanide salts to generate gaseous hydrogen cyanide. The reaction is slower for dry, solid carboxylic acids. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions.
Health Hazard TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Tiglic acid Preparation Products And Raw materials
Preparation Products ALLYL TIGLATE-->BENZYL TIGLATE-->Ethyl tiglate
