Home > Offer to Sell > Intermediates > Agrochemical intermediates > 4-chloro-6-ethyl-5-fluoropyrimidine
4-chloro-6-ethyl-5-fluoropyrimidine
Inquiry
| Post Date: | Mar 03,2015 |
| Expiry Date: | Mar 02,2016 |
| Detailed Description: |
Cas No. :137234-74-3
Quantity: 10Metric Tons What is 4-chloro-6-ethyl-5-fluoropyrimidine? 4-chloro-6-ethyl-5-fluoropyrimidine is a building block and pharmaceutical intermediate for synthetizing bio-active compounds such as broad-spectrum triazole antifungal agents, specifically for producing Voriconazole. Brief introduction of Voriconazole Voriconazole, brand name Vfend, Pfizer, is in the forms of an injectable formulation [200 mg per vial], solid oral formulations as 50 mg and 200 mg tablets and an oral suspension containing 200 mg of voriconazole/5 ml. Voriconazole is a triazole antifungal medication generally used in the treatment of serious invasive fungal infections, including invasive candidiasis, invasive aspergillosis, and certain emerging fungal infections. Patients with these synptoms are generally immunocompromised. Voriconazole can also be used to treat severe invasive fluconazole-resistant Candida infections (including C. krusei) and severe fungal infections with scedosporium spp. and Fusarium spp. Application of 4-chloro-6-ethyl-5-fluoropyrimidine for synthetizing Voriconazole Voriconazole is synthetized in a process of condensing 1-(2,4-difluorophenyl)-2-(1H-1,2,4-triazole-1-yl)ethanone with 4-chloro-6-ethyl-5-fluoropyrimidine, in a ketone, ether, aliphatic hydrocarbon, or aromatic hydrocarbon solvent, to obtain (2R, 3S/2S,3R)-3-(4-chloro-5-fluoropyrimidin-6-yl)-2-(2,4-diflurophenyl)-1-(1H-1,2,4-triazole-1-yl)butan-2-ol. Preparation of 4-chloro-6-ethyl-5-fluoropyrimidine Dissolve 80 g of 6-ethyl~5-fluoro-4-hydroxypyrimidine in 240 ml of dichloromethane to get a solution. Add 78.24 ml of triethylamine to the solution and slowly add 57.4 ml of phosphorus oxychloride thereto for more than 30 minutes. After refluxing the resulting solution for 5 hours, the reaction is completed. Cool the solution to room temperature. After that, add 352 ml of 3N HCl to the solution. The temperature should be maintained at below 200 degrees . Extract the resulting liquid mixture with 100 ml of dichloromethane. Wash the organic layer with 100 ml of water, dry it over magnesium sulfate, and concentrate it under a reduced pressure to obtain the title compound in the form of oil. There is 85.9 g of the product and the yield is 95%. Storage of 4-chloro-6-ethyl-5-fluoropyrimidine: Store under room temperature. |
| CAS Registry Number: | 137234-74-3 |
| Synonyms: | ;6-CHLORO-4-ETHYL-5-FLUORO-1,6-DIHYDROPYRIMIDINE;4-chloro-6-ethyl-5-flouropyrimidine;4-chloro-6-ethyl-5-fluoropyimidine;4-chloro-5-fluoro-6-ethylpyrimidine;V3; |
| Molecular Formula: | C6H6ClFN2 |
| Molecular Weight: | 160.5766 |
| Molecular Structure: | 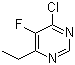
|
| Risk Codes: | 20/21/22-36/37/38:; |
| Safety Description: | 22-36/37/39-45:; |
| Company: | Banff Green Technologies, Inc. [ China ] |
| Contact: | Xue Yang |
| Tel: | +862154795870 |
| Fax: | |
| Email: | banffgreentech@outlook.com |
-
Disclaimer statement:The information and data included above have been realized by the enterprises and compiled by the staff, and are subject to change without notice to you. The Chemnet makes no warranties or representations whatsoever regarding the facticity, accuracy and validity of such information and data. In order to ensure your interest, we suggest you chose the products posted by our gold suppliers or VIP members.