Butafosfan
Inquiry
| Post Date: | Aug 06,2013 |
| Expiry Date: | Feb 02,2014 |
| Detailed Description: |
Cas No. :17316-67-5
CERTIFICATE OF ANALYSIS
Product Name:Butaphosphan Standard:USP30 Batch No.:20110722 Quantity: 50KG Manufacturing Date:Jul.22,2011 Expiry Date:Jul.21,2014 Item Standard Result 1.Characteristics white crystalline powder, slightly hygroscopic. Conforms 2. Solubility Sparingly soluble in water, freely soluble in methanol, ethanol(96%);slightly soluble in acetone; practically insoluble in ether. Conforms 3.Identify (A) IR-Spectrum (B) Reaction of sodium salt Conforms Positive 4 Partical size 80mesh 80mesh 5.Appearance of solution Clear Absorption of sodium salt≤0.05 Conforms 0.03 6.Related Substances Single impurity max 0.2% total impurity max 0.5% 0.12% 0.25% 7.Heavy metal Max 10ppm Conforms 8.Loss on drying Max 0.5% 0.35% 9.Assay 99.0-101.0% 99.31% 10.Conclusion: It complies with the requirements of the USP30 |
| CAS Registry Number: | 17316-67-5 |
| Synonyms: | ;[1-(butylamino)-1-methylethyl](hydroxy)oxophosphonium;Butaphosphan; |
| Molecular Formula: | C7H17NO2P |
| Molecular Weight: | 178.1886 |
| Molecular Structure: | 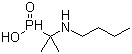
|
| Company: | Hubei MaxSource Chem Co., Ltd. [ China ] |
| Contact: | Bale Yang |
| Tel: | +86-027-83555670 |
| Fax: | 862783555670 |
| Email: | maxsource12@hotmail.com |
-
Disclaimer statement:The information and data included above have been realized by the enterprises and compiled by the staff, and are subject to change without notice to you. The Chemnet makes no warranties or representations whatsoever regarding the facticity, accuracy and validity of such information and data. In order to ensure your interest, we suggest you chose the products posted by our gold suppliers or VIP members.