Home > Offer to Sell > Organic chemicals and Derivatives > Acid, ester and anhydride compounds > Ethylene Glycol
Ethylene Glycol
Inquiry
| Post Date: | Nov 29,2016 |
| Expiry Date: | May 28,2017 |
| Detailed Description: |
Cas No. :107-21-1
Ethylene glycol, also known as "glycol", "ethylene glycol", referred to as the EG. The formula (CH2OH) 2, is the most simple diol. Ethylene glycol is a colorless, odorless, sweet liquid, toxic to animals, the human lethal dose of about 1.6 g / kg. Glycol with water, acetone miscible but less soluble in ethers. Used as a solvent, antifreeze, polyester and synthetic raw materials. Polymers of ethylene glycol polyethylene glycol (PEG) is a phase transfer catalyst, but also for cell fusion; which nitrate is an explosive.
Basic Information Chinese name: Glycol English name: Ethylene glycol Nickname: Glycol, EG, glycol antifreeze, MEG Chemical Properties: Due to its low molecular weight, lively nature, can play esterification / etherification / Aging / oxide / acetal / dehydration reaction. Similar to ethanol, can generate major inorganic or organic acid ester, generally the first and only one hydroxyl group reacts by increasing the temperature, increasing the amount of acid, etc., can make two hydroxyl groups to form an ester. Such as mixed with sulfuric acid and nitric acid, is formed dinitrate. Acid chloride or anhydride of the two hydroxyl groups readily form esters. In ethylene glycol (manganese dioxide, aluminum oxide, zinc oxide or sulfuric acid) heating the catalyst can be intramolecular or intermolecular dehydration. Glycol with an alkali metal or alkaline earth metal alkoxides action. Typically the metal was dissolved in glycols, only one yuan alcohol; salts such as this alkoxide (such as sodium ethylene glycol) was heated to 180 ~ 200 ° C in a stream of hydrogen, to form disodium ethylene glycol and ethylene glycol . Further heating together with ethylene glycol and 2 moles of sodium methylate, disodium ethylene glycol can be obtained. Disodium ethylene glycol is reacted with an alkyl halide to form an ether or di ethylene glycol monomethyl ether. Disodium ethylene glycol and 1,2-dibromoethane reaction, generating dioxane. Furthermore, ethylene glycol is also easily oxidized with the oxidizing agent or a different reaction conditions, can generate a variety of products, such as glycolaldehyde HOCH2CHO, glyoxal OHCCHO, glycolic HOCH2COOH, oxalic HOOCCOOH and carbon dioxide and water. Ethylene glycol and other different periodate oxidation can occur through carbon strand breaks. Applications glycol instead of glycerin can often be used. In leather and pharmaceutical industries, it was used as the solvent and water mixture. Ethylene glycol dinitrate is a derivative of explosives. Ethylene glycol monomethyl or monoethyl ether is a good solvent, such as methyl cellosolve HOCH2CH2OCH3 soluble fibers, resins, paints and many other organic compounds. Glycol solvency is very strong, but it is easily the metabolism of oxygen, generating toxic oxalic acid, and therefore is not widely used as a solvent. Glycol is an antifreeze, 60% ethylene glycol aqueous solution freezes at -40 ° C. Applications: The main system for polyester polyester, polyester resin, dehumidifying agents, plasticizers, surfactants, synthetic fibers, cosmetics and explosives, as a solvent and a dye / ink or the like, preparation of the engine antifreeze, gas dehydration agent, manufacture of resins, can also be used for cellophane, fiber, leather, adhesives humectant. To the production of synthetic resin PET, fiber level PET namely polyester fibers, bottle-grade PET sheet for the production of mineral water bottles. Also the production of alkyd resins, glyoxal, also used as antifreeze. Except as automotive antifreeze, but also for industrial refrigeration transport, it is commonly called coolants. |
| CAS Registry Number: | 107-21-1 |
| Synonyms: | ;1,2-Ethanediol;glycol, 1,2-Dihydroxyethane;Monoethylene glycol;MEG;ethane-1,2-diol;monoethylene-glycol;Ethylene-D4 Glycol-D2;Ethylene glycol-D6; |
| Molecular Formula: | C2H6O2 |
| Molecular Weight: | 62.0837 |
| Molecular Structure: | 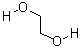
|
| Hazard Symbols: |  Xn:Harmful; Xn:Harmful; |
| Risk Codes: | R22:; |
| Company: | Shijiazhuang Kunmai chemical technology co., LTD. [ China ] |
| Contact: | Winnie |
| Tel: | +86-0311-66686787 |
| Fax: | +86-311-66686787 |
| Email: | kunmaikeji@outlook.com |
-
Disclaimer statement:The information and data included above have been realized by the enterprises and compiled by the staff, and are subject to change without notice to you. The Chemnet makes no warranties or representations whatsoever regarding the facticity, accuracy and validity of such information and data. In order to ensure your interest, we suggest you chose the products posted by our gold suppliers or VIP members.