Neomycin sulfate
Inquiry
| Post Date: | Jun 25,2015 |
| Expiry Date: | Jun 24,2016 |
| Detailed Description: |
Cas No. :1405-10-3
Neomycin sulfate
CAS: 1405-10-3 MF: C23H48N6O17S MW: 712.72 EINECS: 215-773-1 mp >187°C (dec.) refractive index 56 ° (C=10, H2O) storage temp. 2-8°C solubility H2O: 50 mg/mL As a stock solution. Stock solutions should be filter sterilized and stored at 2-8°C. Stable at 37°C for 5 days. Chemical Properties White powder. Usage antibacterial Usage Neomycin is an aminoglycoside antibiotic found in many topical medications. Neomycin has been used as a preventive measure for hepatic encephalopathy and hypercholesterolemia. Neomycin Sulfate Tablets, USP, for oral administration, contain neomycin which is an antibiotic obtained from the metabolic products of the actinomycete Streptomyces fradiae. It has the following molecular formula: C23H46N6O . 2½ H2SO4 with a molecular weight of 614.67. Chemically, it is O-2,6-diamino-2,6-dideoxy-a -D- glucopyranosyl(1®3)-O-b-D- ribofuranosyl-(1®5) O-[2,6-diamino-2,6-dideoxy-a-D-glucopyranosyl-(1®4)]-2-deoxy-D-streptamine. Neomycin B is identical except that the a- D- glucopyranosyl residue in the neobiosamine moiety is b-L-idopyranosyl. SYSTEMIC ABSORPTION OF NEOMYCIN OCCURS FOLLOWING ORAL ADMINISTRATION AND TOXIC REACTIONS MAY OCCUR. Patients treated with neomycin should be under close clinical observation because of the potential toxicity associated with their use. NEUROTOXICITY (INCLUDING OTOTOXICITY) AND NEPHROTOXICITY FOLLOWING THE ORAL USE OF NEOMYCIN SULFATE HAVE BEEN REPORTED, EVEN WHEN USED IN RECOMMENDED DOSES. THE POTENTIAL FOR NEPHROTOXICITY, PERMANENT BILATERAL AUDITORY OTOTOXICITY AND SOMETIMES VESTIBULAR TOXICITY IS PRESENT IN PATIENTS WITH NORMAL RENAL FUNCTION WHEN TREATED WITH HIGHER DOSES OF NEOMYCIN AND/OR FOR LONGER PERIODS THAN RECOMMENDED. Serial, vestibular and audiometric tests, as well as tests of renal function, should be performed (especially in highrisk patients). THE RISK OF NEPHROTOXICITY AND OTOTOXICITY IS GREATER IN PATIENTS WITH IMPAIRED RENAL FUNCTION. Ototoxicity is often delayed in onset and patients developing cochlear damage will not have symptoms during therapy to warn them of developing eighth nerve destruction and total or partial deafness may occur long after neomycin has been discontinued. Neuromuscular blockage and respiratory paralysis have been reported following the oral use of neomycin. The possibility of the occurrence of neuromuscular blockage and respiratory paralysis should be considered if neomycin is administered, especially to patients receiving anesthetics, neuromuscular blocking agents such as tubocurarine, succinylcholine, decamethonium, or in patients receiving massive transfusions of citrate anticoagulated blood. If blockage occurs, calcium salts may reverse these phenomena but mechanical respiratory assistance may be necessary. Concurrent and/or sequential systemic, oral or topical use of other aminoglycosides, including paromomycin and other potentially nephrotoxic and/or neurotoxic drugs such as bacitracin, cisplatin, vancomycin, amphotericin B, polymyxin B, colistin and viomycin, should be avoided because the toxicity may be additive. Other factors which increase the risk of toxicity are advanced age and dehydration. The concurrent use of neomycin with potent diuretics such as ethacrynic acid or furosemide should be avoided, since certain diuretics by themselves may cause ototoxicity. In addition, when administered intravenously, diuretics may enhance neomycin toxicity by altering the antibiotic concentration in serum and tissue. |
| CAS Registry Number: | 1405-10-3 |
| Synonyms: | ;mycifradin sulfate;o-2,6-diamino-2,6-dideoxy-.beta.-l-idopyranosyl-(1.->3)-o-.beta.-d-ribofuranosyl-(1->5))-o-(2,6-diamino-2,6-dideoxy-.alpha.-d-glucopyranosyl-(1->4))-2-deoxy sulfate;Neomycin Sulfate;(1R,2R,3S,4R,6S)-4,6-diamino-2-{[3-O-(2,6-diamino-2,6-dideoxy-beta-L-idopyranosyl)-beta-D-ribofuranosyl]oxy}-3-hydroxycyclohexyl 2,6-diamino-2,6-dideoxy-alpha-D-glucopyranoside sulfate (1:1);(2S,3S,4S,5S,6S)-5-amino-2-(aminomethyl)-6-[(1S,2S,3S,4R,6R)-4,6-diamino-2-[(2R,3S,4R,5S)-4-[(2S,3S,4S,5S,6R)-3-amino-6-(aminomethyl)-4,5-dihydroxy-tetrahydropyran-2-yl]oxy-3-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]oxy-3-hydroxy-cyclohexoxy]tetrahydropyran-3,4-diol,sulfuric acid;Nystatin sulfate;FRADIOMYCIN SULFATE; |
| Molecular Formula: | C23H48N6O17S |
| Molecular Weight: | 712.7222 |
| Molecular Structure: | 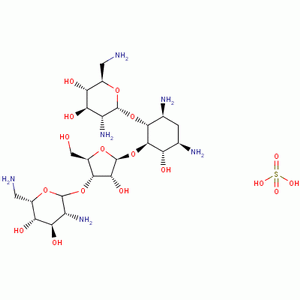
|
| Company: | Hulumimi Pharmaceuticals [ China ] |
| Contact: | Toni Zhong |
| Tel: | 027-50756080 |
| Fax: | 0086-27-88048077-794 |
| Email: | toni@ycgmp.com |
-
Disclaimer statement:The information and data included above have been realized by the enterprises and compiled by the staff, and are subject to change without notice to you. The Chemnet makes no warranties or representations whatsoever regarding the facticity, accuracy and validity of such information and data. In order to ensure your interest, we suggest you chose the products posted by our gold suppliers or VIP members.