trichlorosilane Suppliers, trichlorosilane Manufacturers.
Zibo Linzi Qiquan Industrial Trade Co., Ltd

trichlorosilane
Category :
IntermediatesSynonyms :
trichlorosilane pressure tin;Trichlorosilanecolorlessliq;silicon chloroform;Trichlorosilane,98%;trichlorosilyl;CAS NO :
10025-78-2EC NO :
233-042-5Molecular Formula :
Cl3SiMolecular Weight :
134.4445Main Specifications :
InChI :
InChI=1/Cl3Si/c1-4(2)3Product description :
ZQ-606
Basic information :1. Alias • EnglishChloroform silicon, silicon imitation, trichlorosilane; Trichlorosilane, Silicochloroform.2. UseBasic raw materials for Polysilicon and silane coupling3.Ways of produce:(1) Si and HCl under high temperature reaction.(2) reduction of silicon tetrachloride with hydrogen (using a catalyst of aluminum-containing compounds).characters:1, physical and chemical properties1,1, Molecular weight: 135.431,2, PhysicalMelting point (101.325kPa): -134 ℃; Boiling point (101.325kPa): 31.8 ℃; liquid density (0 ℃): 1350kg/m3; Relative density (gas, air = 1): 4.7; Vapor pressure (-16.4 ℃): 13.3kPa; (14.5 ℃): 53.3kPa; lit: -27.8 ℃; Ignition point: 104.4 ℃; Flash point: -14 ℃; Explosive Limit: 6.9 to 70%;2.toxic levels:1.3, flammability level;1.4, the explosive nature of levels.Chemical Properties
2,1, trichlorosilane at room temperature under normal pressure flow easily with irritating odor colorless volatile liquid. Burn easily in air at -18 ℃ .The following are a fire hazard, stronger in case of the fire is burning, when burning red flame and white smoke is generated, with SiO2, HCl and Cl2 produced: SiHCl3 + O2 → SiO2 + HCl + Cl2;
2,2, trichlorosilane vapor concentration with air to form a wide range of explosive mixture, when heated cause a violent explosion. The thermal stability is of better than dichlorosilane at 900 ℃, decomposition of toxic fumes of chloride (HCl), also generates Cl2 and Si.
2,3, when the smoke case of moisture, with water heated reaction: 2SiHCl3 +3 H2O-→ (HSiO) 2O +6 HCl;
2,4, released in the decomposition of alkaline solution of hydrogen: SiHCl3 +3 NaOH + H2O-→ Si (OH) 4 +3 NaCl + H2;
2,5, An explosive reaction was produced in contact with oxidizing substances . And acetylene, hydrocarbons and other organic hydrocarbons reaction chlorosilane: SiHCl3 + CH ≡ CH 1 → CH2CHSiCl3, SiHCl3 + CH2 = CH2-→ CH3CH2SiCl3
2,6, in the lithium aluminum hydride, lithium boron hydride under the conditions of existence, SiHCl3 can be reduced to silane. when the liquid container has been badly hit SiHCl3 will be on fire. Soluble in benzene, ether. Anhydrous state trichlorosilane on iron and stainless steel does not corrode, but in the presence of water majority of metal will get corrosion.
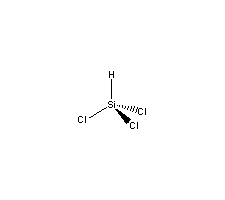
Molecular Structure :
About Us - Top Products- Partner with Us - Contact Us - Help - Sitemap
ChemNet is a registered trademark of Zhejiang NetSun Co., Ltd.
