Home > Offer to Sell > Organic chemicals and Derivatives > Acid, ester and anhydride compounds > Acetic Acid
Acetic Acid
Inquiry
| Post Date: | Sep 07,2014 |
| Expiry Date: | Mar 06,2015 |
| Detailed Description: |
Cas No. :64-19-7
Acetic acid, also known as ethanoic acid, has a molecular formula CH3COOH. It is a weak monoprotic acid which is able to lose a proton from its acid functional group (-COOH) readily and produce a conjugate base, acetate anion. Acetic acid is also a polar protic solvent as it dissolves readily and is miscible in other polar solvents such as water. However, its polar nature makes it insoluble and immiscible in non-polar solvents such as octane. Using acetic acid as a starting reagent, it readily forms other organic reagents such as acetyl chloride and ethanol though substitution and reduction reactions respectively. Acetic acid can also corrode metals such as iron, magnesium and zinc, forming hydrogen gas and metal acetates. But since aluminum forms a protective layer of aluminum oxide on its surface, it is acid-resistance and hence aluminum tanks are used as an option to transport acetic acid. Alternatively, high-density polyethylene (HDPE) drums can also be used to transport acetic acids as due to their resistivity. Manufacturing Process Carbonylation of Methanol Acetic acid is manufactured via carbonylation of methanol. Metal carbonyl is added to methanol via rhodium-catalyzed Monsanto process or iridium-catalyzed Cativa process. However, with the introduction of iridium-catalyzed Cativa process, Monsanto process became obsoleted. Due to the economical and environmental friendliness, Cativa process soon became the main process used to manufacture acetic acid. Acetaldehyde Oxidation Acetic acid is also manufactured via acetaldehyde oxidation, where oxidizing butane and hydrating ethylene via Wacker process obtain acetaldehyde. This crude acetaldehyde is purified by extractive distillation, followed by fractional distillation. This acetaldehyde will further oxidize to acetic acid. Oxidative Fermentation and Anaerobic Fermentation Acetic acid can also be manufactured via oxidative fermentation using acetic acid bacteria Acetobacter in alcoholic content, and via anaerobic fermentation using anaerobic bacteria Acetobacterium. The Acetobacter method remains more cost effective to produce acetic acid. Textile Industry Acetic acids are used for textile processing and printing. During the dyeing process, acetic acid plays a vital role in maintaining the accurate and constant pH required for dyeing bath and aids in the absorption of dyes by textile fiber. |
| CAS Registry Number: | 64-19-7 |
| Synonyms: | ;Glacial acetic acid;Acetic Acid Glacial BP;Natural Acetic Acid;Acetic Acid Glacial;GAA;Acetic Acid, Glacial;Ethanoic acid;Acetasol; |
| Molecular Formula: | C2H4O2 |
| Molecular Weight: | 60.05 |
| Molecular Structure: | 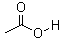
|
| Hazard Symbols: | 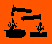 C:Corrosive; C:Corrosive; |
| Risk Codes: | R10:; R35:; |
| Safety Description: | S23:; S26:; S45:; |
| Company: | Tradeasia International DMCC [ United Arab Emirates ] |
| Contact: | Leah Padilla |
| Tel: | +971-4-2778045 |
| Fax: | |
| Email: | leahdmcc@chemtradeasia.com |
-
Disclaimer statement:The information and data included above have been realized by the enterprises and compiled by the staff, and are subject to change without notice to you. The Chemnet makes no warranties or representations whatsoever regarding the facticity, accuracy and validity of such information and data. In order to ensure your interest, we suggest you chose the products posted by our gold suppliers or VIP members.