Barium Carbonate
Inquiry
| Post Date: | Oct 11,2018 |
| Expiry Date: | Oct 11,2019 |
| Detailed Description: |
Cas No. :513-77-9
Specs:99% min Payment Method: TT,LC,DP,DA Barium Carbonate (BaCO3) CAS No.: 513-77-9 Lower Fe/S as special industry request Micro/Light grade/Free flowing/Granular Barium carbonate (BaCO3), also known as witherite, is a chemical compound used in rat poison, bricks, ceramic glazes and cement. Witherite crystallizes in the orthorhombic system. The crystals are invariably twinned together in groups of three, giving rise to pseudo-hexagonal forms somewhat resembling bipyramidal crystals of quartz, the faces are usually rough and striated horizontally. Witherite The mineral is named after William Withering, who in 1784 recognized it to be chemically distinct from barytes. It occurs in veins of lead ore at Hexham in Northumberland, Alston in Cumbria, Anglezarke, near Chorley in Lancashire and a few other localities. Witherite is readily altered to barium sulfate by the action of water containing calcium sulfate in solution and crystals are therefore frequently encrusted with barytes. It is the chief source of barium salts and is mined in considerable amounts in Northumberland. It is used for the preparation of rat poison, in the manufacture of glass and porcelain, and formerly for refining sugar. It is also used for controlling the chromate to sulfate ratio in chromium electroplating baths. China Barium Carbonate price BaCO3 CAS No.: 513-77-9 Lower Fe/S as special industry request Micro/Light grade/Free flowing/Granular Barium carbonate (BaCO3), also known as witherite, is a chemical compound used in rat poison, bricks, ceramic glazes and cement. Witherite crystallizes in the orthorhombic system. The crystals are invariably twinned together in groups of three, giving rise to pseudo-hexagonal forms somewhat resembling bipyramidal crystals of quartz, the faces are usually rough and striated horizontally. Witherite The mineral is named after William Withering, who in 1784 recognized it to be chemically distinct from barytes. It occurs in veins of lead ore at Hexham in Northumberland, Alston in Cumbria, Anglezarke, near Chorley in Lancashire and a few other localities. Witherite is readily altered to barium sulfate by the action of water containing calcium sulfate in solution and crystals are therefore frequently encrusted with barytes. It is the chief source of barium salts and is mined in considerable amounts in Northumberland. It is used for the preparation of rat poison, in the manufacture of glass and porcelain, and formerly for refining sugar. It is also used for controlling the chromate to sulfate ratio in chromium electroplating baths. |
| CAS Registry Number: | 513-77-9 |
| Synonyms: | C.I. 77099;C.I. Pigment White 10;barium carbonate, natural;barium carbonate, other than natural;Barium Carbonate (Powder);Barium Carbonate (Low Sulphur);BariumcarbonateCaSrmeshwhitepowder;Bariumcarbonatewhitepowder;Barium carbonate,( 99.9%-Ba;Barium carbonate 99+ %;Barium Carbonate (free flowing);Barium carbonate,high purity |
| Molecular Formula: | CBaO3 |
| Molecular Weight: | 197.337 |
| Molecular Structure: | 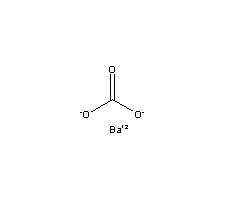
|
| Hazard Symbols: |  Xn:Harmful; Xn:Harmful; |
| Risk Codes: | R22:; |
| Safety Description: | S24/25:; |
| Company: | Sinoright International Trade Co. Ltd. [ China ] |
| Contact: | Harry Du |
| Tel: | 0086-411-86596755 39210844 130 |
| Fax: | 0086-411-86596060 39210855 |
| Email: | harry.du@sinoright.net |
-
Disclaimer statement:The information and data included above have been realized by the enterprises and compiled by the staff, and are subject to change without notice to you. The Chemnet makes no warranties or representations whatsoever regarding the facticity, accuracy and validity of such information and data. In order to ensure your interest, we suggest you chose the products posted by our gold suppliers or VIP members.

-
- Sulfanilamide
- m-Methyl Benzonic Acid
- N,O-Bis(trimethylsilyl)ace...
- Methanesulfonyl chloride
- zirconium acetate
- zircon isocaprylate
- DISPERSE RED 73
- DISPERSE VIOLET 57
- DISPERSE VIOLET 77
- DISPERSE YELLOW 211
- FAST RED B BASE
- zirconium sulphate
- CATIONIC BLACK FDL
- DISPERSE RED 152
- Levamisole
- Levofloxacin
- Lidocaine HCl
- Lincomycin HCl
- Lomefloxacin HCl
- Indomethacin
- Isotretinoin
- Ivermectine
- Ketoprofen
- Ketotifen Fumarate
- Lorsartan Potassium