Home > Offer to Sell > Intermediates > Pharmaceutical intermediates > Stearic Acid CAS 57-11-4 (jerryzhang001@chembj.com)
Stearic Acid CAS 57-11-4 (jerryzhang001@chembj.com)
Inquiry
| Post Date: | Mar 24,2017 |
| Expiry Date: | Mar 24,2018 |
| Detailed Description: |
Cas No. :57-11-4
Stearic Acid CAS 57-11-4
Product Name: Stearic acid; Synonyms: CETYLACETIC ACID;FEMA 3035;CARBOXYLIC ACID C18;C18;C18:0 FATTY ACID;ACIDUM STEARICUM 50;N-OCTADECYLIC ACID;N-OCTADECANOIC ACID; CAS: 57-11-4; MF: C18H36O2; MW: 284.48; EINECS: 266-928-5; Product Categories: Miscellaneous Natural Products;Alkylcarboxylic Acids;Biochemistry;Higher Fatty Acids & Higher Alcohols;Monofunctional & alpha,omega-Bifunctional Alkanes;Monofunctional Alkanes;Saturated Higher Fatty Acids;Chemical intermediate;plasticizer, stabilizer and lubricant;Food additives; Melting point: 67-72 °C(lit.); Boiling point: 361 °C(lit.); density: 0.84; vapor pressure: 1 mm Hg ( 173.7 °C); Fp: >230 °F; storage temp.: 2-8°C; HS Code : 38231100; Description: Stearic acid is one of several major long-chain fatty acids comprising oils and fats. It is presented in animal fats, oil and some kinds of vegetable oils as wellin the form of glycerides. These oils, after hydrolysis, produce the stearic acid. Stearic acid is a fatty acid widely existing in nature and has the general chemical properties of carboxylic acids. Almost all kinds of fat and oil contain certain amount of stearic acid with the content in the animal fats being relative high. For example, the content in the butter can reach up to 24% while the content in vegetable oil is relative low with the value in tea oil being 0.8% and the oil in palm being 6%. However, the content in cocoa can reach as high as 34%. Stearic acid is mainly used for the production of stearates such as sodium stearate, magnesium stearate, calcium stearate, lead stearate, aluminum stearate, cadmium stearate, iron stearate, and potassium stearate. Usage: In general, the applications of stearic acid exploit its bifunctional character, with a polar head group that can be attached to metal cations and a nonpolar chain that confers solubility in organic solvents. The combination leads to uses as a surfactant and softening agent. Stearic acid undergoes the typical reactions of saturated carboxylic acids, a notable one being reduction to stearyl alcohol, and esterification with a range of alcohols. This is used in a large range of manufactures, from simple to complex electronic devices. Soaps, cosmetics, detergents Stearic acid is mainly used in the production of detergents, soaps, and cosmetics such as shampoos and shaving cream products. Soaps are not made directly from stearic acid, but indirectly by saponification of triglycerides consisting of stearic acid esters. Esters of stearic acid with ethylene glycol, glycol stearate, and glycol distearate are used to produce a pearly effect in shampoos, soaps, and other cosmetic products. They are added to the product in molten form and allowed to crystallize under controlled conditions. Detergents are obtained from amides and quaternary alkylammonium derivatives of stearic acid. Lubricants, softening and release agents In view of the soft texture of the sodium salt, which is the main component of soap, other salts are also useful for their lubricating properties. Lithium stearate is an important component of grease. The stearate salts of zinc, calcium, cadmium, and lead are used to soften PVC. Stearic acid is used along with castor oil for preparing softeners in textile sizing. They are heated and mixed with caustic potash or caustic soda. Related salts are also commonly used as release agents, e.g. in the production of automobile tires. Niche uses Being inexpensively available and chemically benign, stearic acid finds many niche applications, for example, in making plaster castings from a plaster piece mold or waste mold and in making the mold from a shellacked clay original. In this use, powdered stearic acid is mixed in water and the suspension is brushed onto the surface to be parted after casting. This reacts with the calcium in the plaster to form a thin layer of calcium stearate, which functions as a release agent. When reacted with zinc it forms zinc stearate, which is used as a lubricant for playing cards (fanning powder) to ensure a smooth motion when fanning. In compressed confections, it is used as a lubricant to keep the tablet from sticking to the die. Stearic acid is also used as a negative plate additive in the manufacture of lead-acid batteries. It is added at the rate of 0.6 g per kg of the oxide while preparing the paste.[11] It is believed to enhance the hydrophobicity of the negative plate, particularly during dry-charging process. It also reduces the extension of oxidation of the freshly formed lead (negative active material) when the plates are kept for drying in the open atmosphere after the process of tank formation. As a consequence, the charging time of a dry uncharged battery during initial filling and charging (IFC) is comparatively lower, as compared to a battery assembled with plates which do not contain stearic acid additive. Fatty acids are classic components of candle-making. Stearic acid is used along with simple sugar or corn syrup as a hardener in candies. Stearic acid is used to produce dietary supplements. In fireworks, stearic acid is often used to coat metal powders such as aluminium and iron. This prevents oxidation, allowing compositions to be stored for a longer period of time. Stearic acid is a common lubricant during injection molding and pressing of ceramic powders.[12] It is also used as a mold release for foam latex that is baked in stone molds. |
| CAS Registry Number: | 57-11-4 |
| Synonyms: | ;Octadecanoic acid;n-Octadecan acid;Octadecan acid; |
| Molecular Formula: | C18H36O2 |
| Molecular Weight: | 284.48 |
| Molecular Structure: | 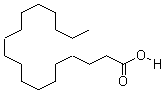
|
| Hazard Symbols: |  F:Flammable; F:Flammable; Xi:Irritant; Xi:Irritant; |
| Risk Codes: | R11:; R36/37/38:; |
| Safety Description: | S16:; S26:; S37/39:; |
| Company: | Nanjing Bangnuo Biotechnology Co., Ltd. [ China ] |
| Contact: | Jerry Zhang |
| Tel: | 86-25-52198306 |
| Fax: | 86-25-52198306 |
| Email: | jerryzhang001@chembj.com |
-
Disclaimer statement:The information and data included above have been realized by the enterprises and compiled by the staff, and are subject to change without notice to you. The Chemnet makes no warranties or representations whatsoever regarding the facticity, accuracy and validity of such information and data. In order to ensure your interest, we suggest you chose the products posted by our gold suppliers or VIP members.