Vonoprazan Fumarate
Inquiry
| Post Date: | Sep 25,2025 |
| Expiry Date: | Mar 24,2026 |
| Detailed Description: |
Cas No. :1260141-27-2
Vonoprazan fumarate (Takecab), discovered and developed by Takeda and Otsuka, was approved by the PMDA of Japan in December 2014, and is indicated for the treatment of gastric ulcer, duodenal ulcer and reflux esophagitis.
Vonoprazan fumarate is an oral, newly synthesised potassium-competitive acid blocker (P-CAB) with antisecretory activity. It is also a proton pump inhibitor (PPI) reversibly inhibiting H+/K+, ATPase. It is mainly used in the treatment of acid-related diseases such as GERD and peptic ulcer disease. |
| CAS Registry Number: | 1260141-27-2 |
| Synonyms: | ;Vonoprazan fumarate;TAK-438;TAK 438;5-(2-Fluorophenyl)-N-methyl-1-(3-pyridinylsulfonyl)-1H-pyrrole-3-methanamine (2E)-2-butenedioate;Vonaprazan;TAK438; |
| Molecular Formula: | C21H20FN3O6S |
| Molecular Weight: | 461.46300 |
| Molecular Structure: | 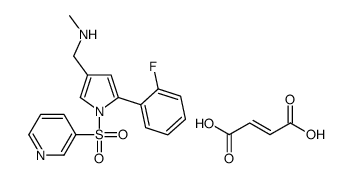
|
| Company: | Sinoway Industrial Co.LTD. [ China ] |
| Contact: | Xie Wen Feng |
| Tel: | +86-592-5881089 |
| Fax: | +86-592-5854960 |
| Email: | xie@china-sinoway.com |
-
Disclaimer statement:The information and data included above have been realized by the enterprises and compiled by the staff, and are subject to change without notice to you. The Chemnet makes no warranties or representations whatsoever regarding the facticity, accuracy and validity of such information and data. In order to ensure your interest, we suggest you chose the products posted by our gold suppliers or VIP members.

-
- Clarithromycin
- Polymyxin B Sulfate
- Progesterone
- Vortioxetine Hydrobromide
- Piperacillin Sodium an...
- gentamycin sulphate
- Captopril
- esomeprazole sodium
- Glibenclamide
- Chondroitin sulfate
- Coenzyme Q10
- Biotin
- Matrixyl
- Acetyl Hexapeptide-3
- Epimedium Extract
- Acerola Extract
- 3-Amino-1-adamantanol
- L-Prolinamide
- (1S,3S,5S)-2-Azabicyclo[3....
- (2S)-1-chloroacetyl-2-pyrr...
- (1S,3S,5S)-2-Azabicyclo[3....
- Rosuvastatin Ethyl ester
- 2,6-Dihydroxy-3-methylpuri...
- 2,3,4,6-Tetrakis-O-trimeth...
- 3-(trifluoromethyl)-5,6,7,...