Home > Offer to Sell > Pharmaceuticals and Biochemicals > Antianaphylaxis(antihistamines) and antidotes > ceftriaxone sodium
ceftriaxone sodium
Inquiry
| Post Date: | Jan 04,2017 |
| Expiry Date: | Jan 04,2018 |
| Detailed Description: |
Cas No. :74578-69-1
Payment Method: T/T Approved by National GMP certificate, approved by FDA and COS Specification:0.5g,1.0g,2.0g,2.5g Dosage form: powder Packaging: vial/box/carton Payment Terms: T/T Our factory not only specialized in producing various kinds of powder injections but also water injections, Tablets, Capsules, pharmaceutical raw materials… Such as benzylpenicillin sodium for injection, ampicillin sodium for injection, amoxicillin sodium and clavulanate potassium injection…… Welcome to visit our factory to discuss business. Jenny Shandong Weifang Pharmaceutical Factory Co., Ltd E-mail: kindmind1988@hotmail.com |
| CAS Registry Number: | 74578-69-1 |
| Synonyms: | ;CeftriaxoneSodium;disodium (6R,7R)-7-{[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetyl]amino}-3-{[(2-methyl-6-oxido-5-oxo-2,5-dihydro-1,2,4-triazin-3-yl)sulfanyl]methyl}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate;Ceftiaxone Sodium; |
| Molecular Formula: | C18H16N8Na2O7S3 |
| Molecular Weight: | 598.5436 |
| Molecular Structure: | 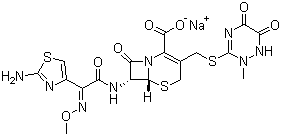
|
| Company: | SHIJIAZHUANG KANGMIN I/E CO.,LTD [ China ] |
| Contact: | jenny |
| Tel: | +86-311-85362019 |
| Fax: | +86-311-85362019 |
| Email: | jxh319@126.com |
-
Disclaimer statement:The information and data included above have been realized by the enterprises and compiled by the staff, and are subject to change without notice to you. The Chemnet makes no warranties or representations whatsoever regarding the facticity, accuracy and validity of such information and data. In order to ensure your interest, we suggest you chose the products posted by our gold suppliers or VIP members.